Since their discovery in the 1980s, quantum dots have been widely used in various fields such as displays. A collaborative effort between the research groups of Professor Wang Ligang (Distinguished Researcher/Assistant Professor), Professor Zhou Huanping (Professor), Academician Yan Chunhua/Professor Sun Lingdong (College of Chemistry and Molecular Engineering), and Sir Richard Friend (College of the Cavendish Laboratory, University of Cambridge), has achieved highly efficient light-emitting diodes (LEDs) with varying numbers of quantum dots per atomic layer.
The electroluminescence peak of this LED is controllably tunable in the range of 607–728 nm, and it achieves an external quantum efficiency (EQE) of 26.8%. This achievement is of great significance to the field of high-definition displays and was published on February 14, 2025, in the internationally renowned journal Science Advances, entitled "Efficient perovskite LEDs with tailored atomic layer number emission at fixed wavelengths" (D125).
In 2023, the Nobel Prize in Chemistry was awarded to three scientists who invented colloidal quantum dots. The wide color gamut, narrow emission peak, and high external quantum efficiency (EQE) of quantum dots are core requirements for next-generation high-definition display technologies.
Traditional quantum dot color modulation is mainly achieved by controlling the size of the quantum dots. However, the size of quantum dots is easily affected by synthesis conditions such as precursor composition, reaction temperature/time, and ligand type/ratio, resulting in poor repeatability of emission wavelengths. Lead halide perovskite quantum dot LEDs have attracted widespread attention from academia and industry due to their ultra-high efficiency, solution-preparable nature, and ability to cover emission wavelengths from blue to infrared.
However, in previous research on perovskite LEDs, mixed halogens were usually used to adjust the band gap to regulate the emission wavelength. But under photoexcitation or electroluminescence conditions, mixed halide perovskite materials are prone to component segregation, resulting in unstable emission color.
Furthermore, the presence of the energy funnel effect means that in multi-n-value (multi-bandgap) perovskite systems, luminescence often occurs in the large-n-value phase with small bandgap and long wavelength. Förster resonance energy transfer and charge transfer are considered to be two possible mechanisms causing this effect, but the exact mechanism of the energy funnel effect and the differences between the two in photoluminescence and electroluminescence are still not fully understood.
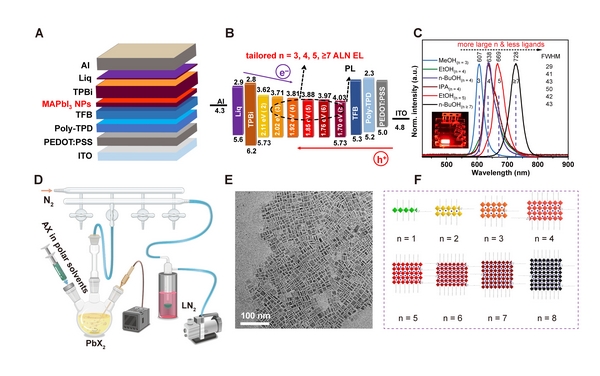
MAPbI3 perovskite quantum dot LEDs with different atomic layer numbers
To address these challenges, Wang Ligang et al. proposed a novel approach to control the electroluminescence wavelength of LEDs based on the atomic number of quantum dots. This approach relies on a newly developed nanomaterial synthesis method—"Rapid Evaporation Polar Solvent-Assisted Perovskite Quantum Dot Synthesis (FEPS)." This method involves injecting a polar solvent (water, alcohol) solution of a perovskite A-site cation precursor into a lead halide solution, followed by rapid removal of the polar solvent using vacuum flash evaporation, thereby obtaining high-quality perovskite quantum dots. By using different polar solvents and post-processing methods, perovskite quantum dot solutions with different atomic numbers can be obtained, leading to the fabrication of LEDs with different emission wavelengths.
Based on this synthesis method, the research team achieved for the first time precise control of electroluminescence (EL) emission from perovskite LEDs with 3, 4, 5, and 7 atomic layers of perovskite quantum dots, obtaining LEDs with main emission peaks of 607, 638, 669, and 728 nm, respectively, to meet the requirements of different technical standards for the wavelengths of the three primary colors of red light. More importantly, because the emission wavelength of this LED depends on the integer number of atomic layers, rather than the size or composition that is easily affected by the preparation conditions, its emission wavelength exhibits high repeatability. The wavelength difference between different batches of LEDs is less than 1 nm, which is significantly better than traditional bulk quasi-two-dimensional perovskite LEDs (whose wavelength difference can reach 40 nm).
Furthermore, the color purity (half-width at half maximum of 29–43 nm) of this type of quantum dot LED is significantly superior to that of traditional bulk quasi-two-dimensional perovskite materials (half-width at half-width of 61 nm). This LED achieves an external quantum efficiency of 26.8%, and the device exhibits excellent operational and color stability, demonstrating great application potential in next-generation ultra-high-definition display technologies.
Carrier dynamics studies have shown that charge transfer is the primary energy transfer pathway under perovskite LED operating conditions. In this quantum dot LED, due to slower charge transfer and a lower Förster resonance energy transfer probability, electroluminescence exhibits a significant blue shift compared to photoluminescence, indicating that more photons are emitted from quantum dots with small n values. This carrier dynamics study also resolves the debate surrounding the energy transfer mechanism of the funnel effect caused by Förster resonance energy transfer or charge transfer in multi-n value systems, providing important theoretical guidance for the design of quantum dot LEDs.

Performance of LEDs with different numbers of atomic layers
The first authors of the paper are Wang Ligang, Zher Ying Ooi (a PhD student at the University of Cambridge), and Jia Fengyan (a PhD graduate from Yan Chunhua's research group). Wang Ligang, Zhou Huanping, Sun Lingdong, Yan Chunhua, and Richard Friend are the co-corresponding authors. Other collaborators are from the University of Cambridge, Zhejiang University, and other institutions. This research was supported by the Royal Society Newton International Fellowship and jointly funded by the National Natural Science Foundation of China, the Ministry of Science and Technology, the Beijing Municipal Natural Science Foundation, and the European Research Council. (Image source: Peking University Shenzhen Graduate School)


